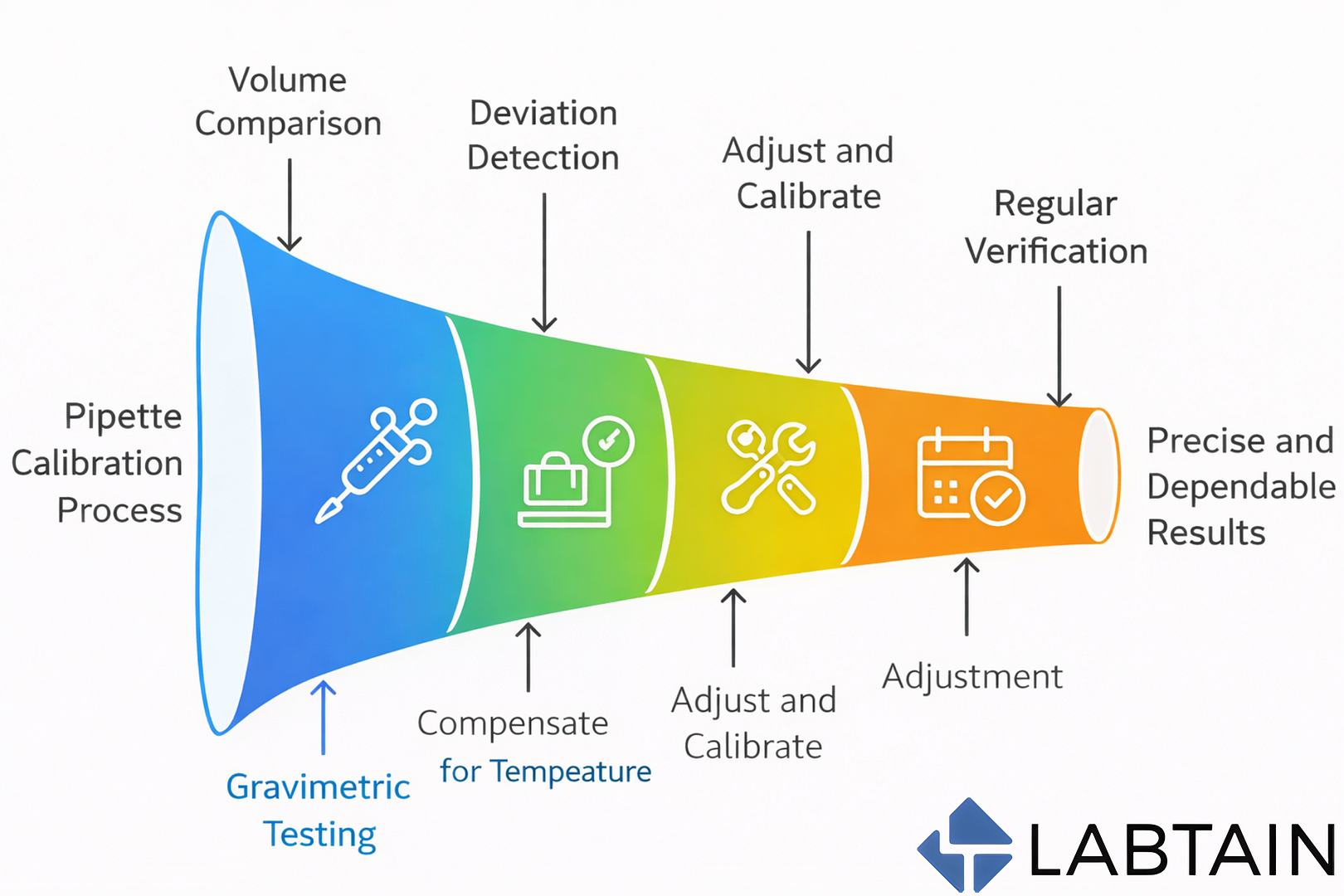
Accurate pipetting is fundamental to reliable laboratory results. Even small volume deviations can lead to failed experiments, irreproducible data, and regulatory non-compliance. This is why pipette calibration is not optional—it is a core quality control requirement.
This article explains how often pipettes should be calibrated, what ISO 8655 actually requires, and how laboratories can establish a practical, defensible calibration schedule.
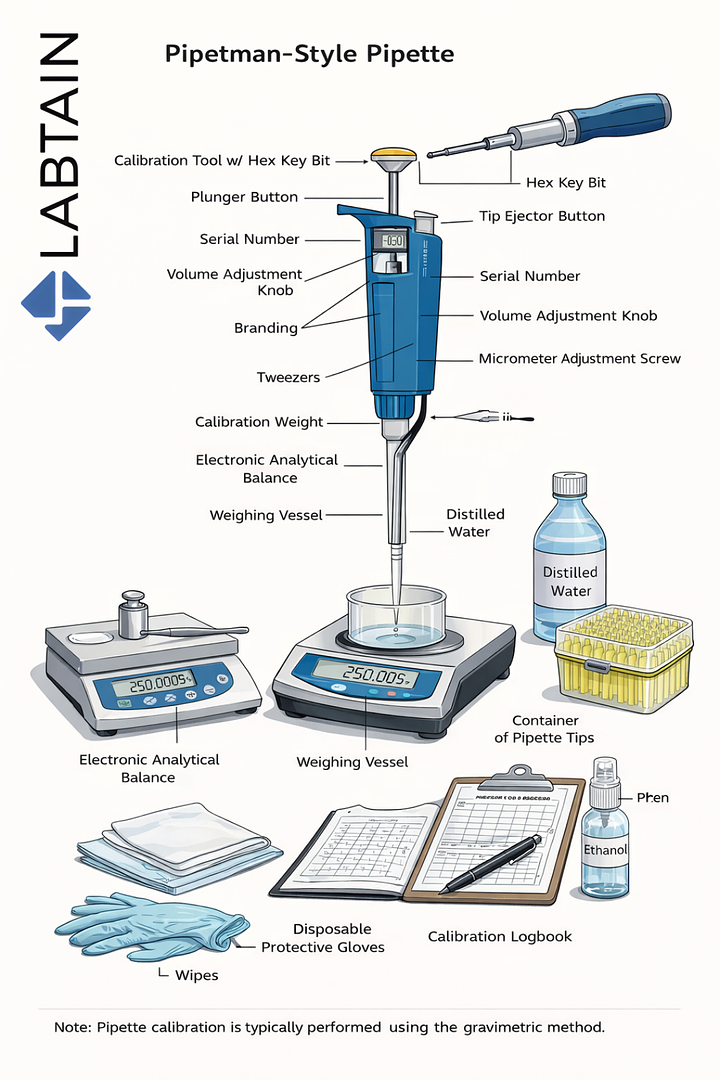
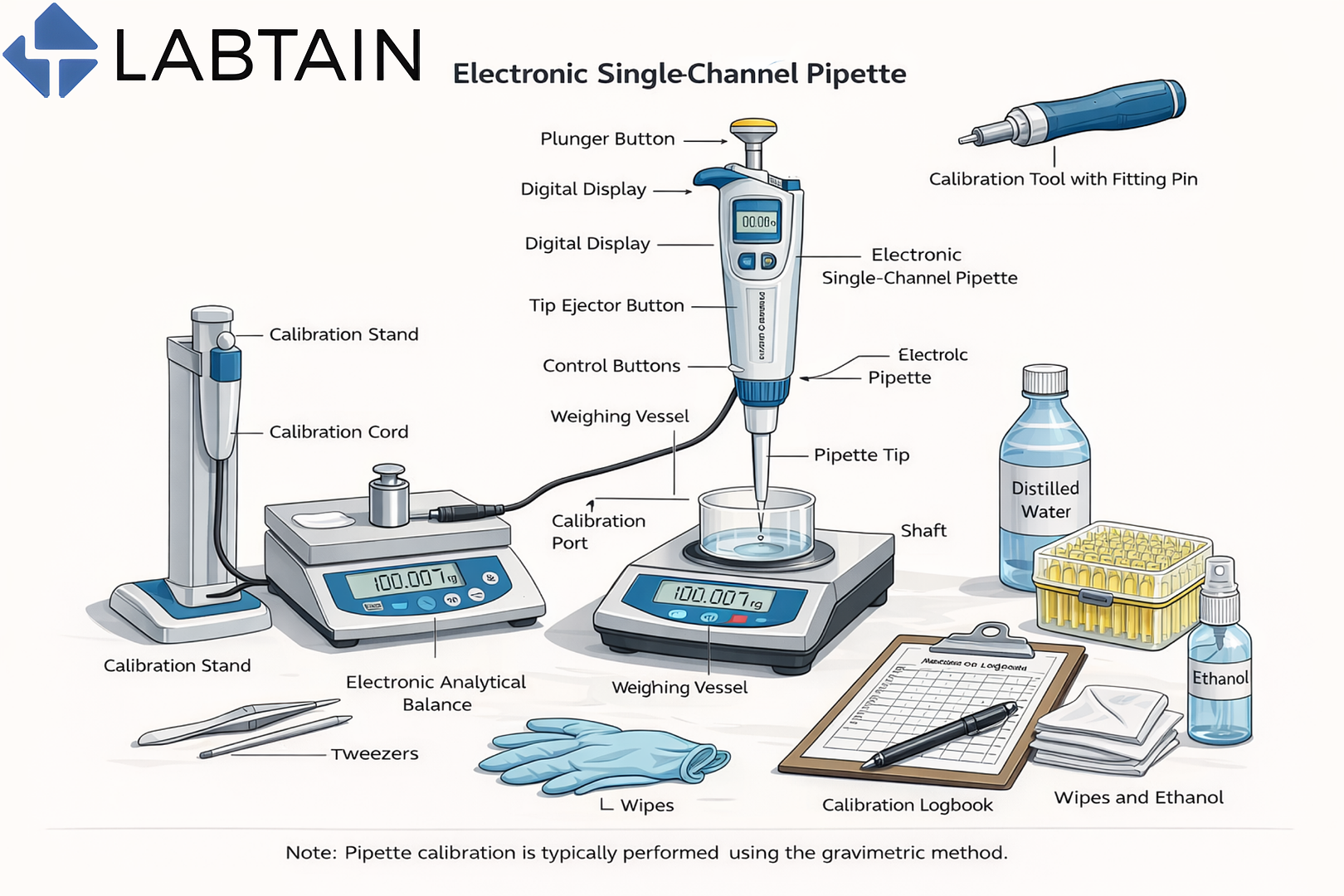
What Is Pipette Calibration?
Pipette calibration is the process of verifying that a pipette dispenses liquid volumes accurately and precisely within defined tolerance limits. In most laboratories, this is done using the gravimetric method, where dispensed liquid is weighed on an analytical balance and compared against expected values.
Calibration answers two critical questions:
- Is the pipette delivering the correct volume (accuracy)?
- Is it delivering that volume consistently (precision)?
What Is ISO 8655?
ISO 8655 is the international standard governing piston-operated volumetric apparatus, including:
- Single-channel pipettes
- Multi-channel pipettes
- Electronic pipettes
- Positive displacement pipettes
It defines:
- Test methods
- Environmental conditions
- Measurement procedures
- Maximum permissible errors (MPE)
Importantly, ISO 8655 does not mandate a fixed calibration interval. Instead, it places responsibility on the laboratory to establish a justified schedule based on risk and usage.
How Often Should Pipettes Be Calibrated?
While ISO 8655 does not specify an exact frequency, industry best practice has converged on the following guidance.
Standard Recommendation
Every 6–12 months
This interval is widely accepted by:
- Research laboratories
- Universities
- Biotech companies
- QA auditors
However, this is only a baseline.
Calibration Frequency Based on Usage
High-Use Pipettes
Every 3–6 months
Examples:
- Daily use
- High-throughput labs
- Clinical or production environments
Rationale:
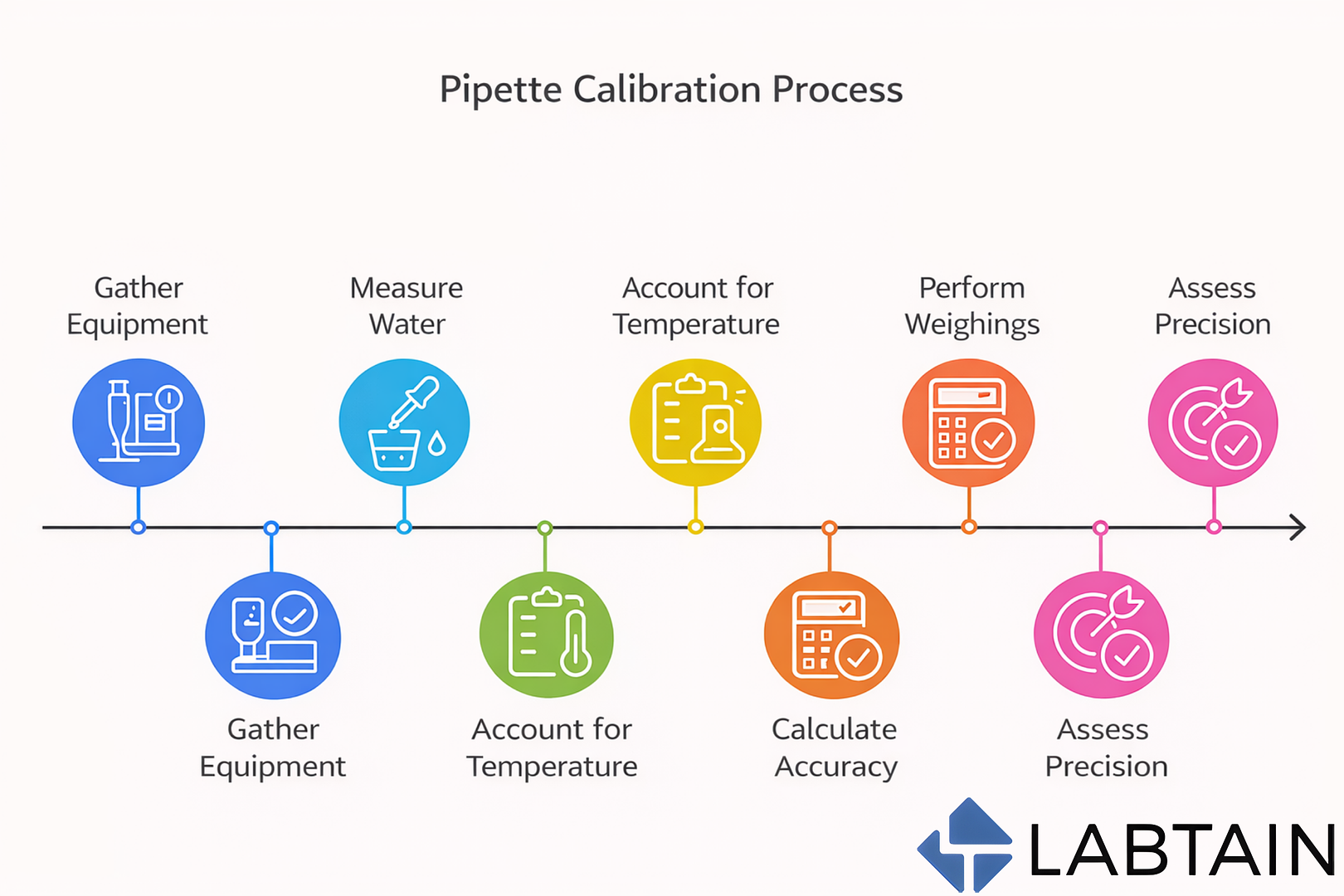
Frequent mechanical wear and repeated plunger cycles increase drift risk.
Moderate-Use Pipettes
Every 6–12 months
Examples:
- Academic research labs
- General R&D
- Teaching laboratories
This is the most common calibration interval.
Low-Use Pipettes
Every 12 months
Examples:
- Backup pipettes
- Specialty volume ranges
- Infrequently used instruments
Even unused pipettes can drift due to seal aging and environmental factors.
Calibration Frequency Based on Risk
ISO 8655 encourages a risk-based approach.
You should calibrate more frequently if:
- Results affect regulatory submissions
- Data supports clinical or GMP processes
- Pipettes are used for critical assays
- Results must be legally defensible
You may justify longer intervals if:
- Pipettes are rarely used
- Historical calibration data shows stability
- Usage is non-critical
Events That Require Immediate Recalibration
Regardless of your normal schedule, recalibration is required if:
- The pipette is dropped
- The pipette is repaired or adjusted
- Performance issues are suspected
- Inconsistent results appear
- The lab environment changes significantly
Skipping recalibration in these cases is a common audit finding.
ISO 8655 Environmental Requirements
Calibration must be performed under controlled conditions:
- Temperature: typically 20–25°C
- Relative humidity: stable and recorded
- Evaporation control in place
- Calibrated analytical balance used
Failure to meet environmental requirements can invalidate results—even if the pipette itself is functional.
Calibration vs Adjustment (Critical Distinction)
- Calibration: Measurement and documentation only
- Adjustment: Mechanical change to correct performance
ISO 8655 requires calibration, but adjustment should only be performed when results exceed tolerance.
Every adjustment must be followed by a full recalibration.
Calibration Certificates: What to Look For
A compliant calibration certificate should include:
- Pipette identification (model, serial number)
- Test volumes
- Accuracy and precision results
- ISO 8655 reference
- Environmental conditions
- Date and technician identification
- Traceability statement
Missing or vague certificates are a red flag during audits.
In-House vs Third-Party Calibration
In-House Calibration
Advantages:
- Faster turnaround
- Lower long-term cost
- Immediate verification
Requirements:
- Analytical balance
- Controlled environment
- Trained personnel
- Documented procedures
Third-Party Calibration
Advantages:
- External validation
- Reduced internal workload
- Audit-friendly documentation
Trade-offs:
- Downtime
- Recurring cost
- Scheduling delays
Many labs use a hybrid approach depending on criticality.
Used and Refurbished Pipettes: Calibration Matters More
When purchasing used pipettes:
- Factory calibration may no longer be valid
- Shipping and storage can affect seals
- Verification is essential before use
A professionally refurbished pipette should be:
- Cleaned
- Functionally tested
- Calibration-verified
- Accompanied by documentation
Key Takeaways
- ISO 8655 does not mandate a fixed interval
- 6–12 months is the industry baseline
- High-use and high-risk pipettes require more frequent calibration
- Environmental control and documentation are as important as measurement
- Calibration protects data integrity, compliance, and research credibility
Final Recommendation
Do not ask, “How often is calibration required?”
Ask, “How often can we defend our calibration interval during an audit?”
That mindset aligns with ISO 8655, good science, and professional lab management.